Chandkheda, Ahmedabad, Gujarat
- GST NO. : 24CJWPK9058H1ZI
| Business Type | Exporter, Supplier, Trader, Distributor |
| Form | Celon |
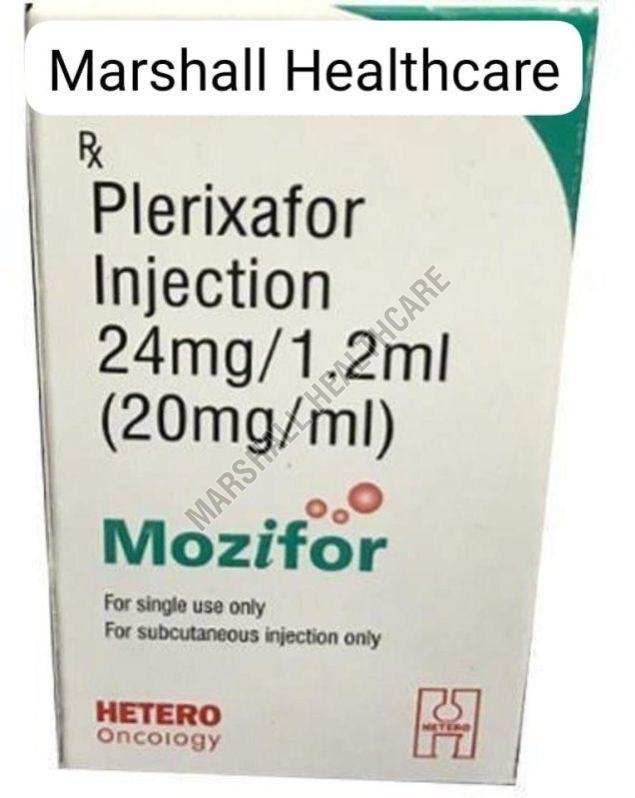
| Brand Name | Plerixafor Injection |
| Dosage Form | Injection |
| Click to view more | |
Product Details
Plerixafor, sold under the brand name Mozobil, is a medication used in conjunction with other drugs to help mobilize hematopoietic stem cells (HSCs) from the bone marrow into the peripheral bloodstream. These stem cells are then collected from the blood and used for an autologous stem cell transplant.
Uses
Plerixafor is used in patients with certain types of cancer, specifically non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM). It is given as part of a regimen to prepare the patient for an autologous stem cell transplant, where the patient's own stem cells are collected and then reinfused after high-dose chemotherapy or radiation.
Plerixafor is used in combination with a granulocyte colony-stimulating factor (G-CSF) medication, such as filgrastim (Neupogen), which stimulates the bone marrow to produce more stem cells. Plerixafor's role is to help these newly produced stem cells move from the bone marrow into the bloodstream so they can be collected through a procedure called apheresis. This is particularly useful in patients who have a history of poor stem cell mobilization with G-CSF alone.
Mechanism of Action
Plerixafor is classified as a hematopoietic stem cell mobilizer and a CXCR4 antagonist. It works by blocking the binding of a protein called stromal cell-derived factor-1 alpha (SDF-1α) to its receptor, CXCR4, which is found on the surface of hematopoietic stem cells. The natural interaction between SDF-1α and CXCR4 is what anchors the stem cells within the bone marrow. By disrupting this interaction, plerixafor causes the stem cells to be released into the bloodstream, where they can be collected.
Administration
Plerixafor is administered as a subcutaneous (under the skin) injection by a healthcare professional in a medical facility. The injection is typically given once a day, approximately 11 hours before the apheresis procedure, for up to four days. The dosage is based on the patient's body weight and is part of a carefully managed treatment schedule.
Side Effects
As with any powerful medication, plerixafor can cause side effects. Some common side effects include:
-
Gastrointestinal issues: Nausea, vomiting, diarrhea, gas, and stomach pain.
-
Fatigue and headache.
-
Dizziness and joint pain.
-
Reactions at the injection site: Pain, redness, or swelling.
-
Vasovagal reactions: Some patients may experience fainting or lightheadedness, particularly shortly after the injection.
More serious, but less common, side effects can occur, including:
-
Splenic enlargement and rupture: Plerixafor, in combination with G-CSF, can cause the spleen to enlarge, and in rare cases, rupture. Patients are advised to seek immediate medical attention if they experience pain in the upper left abdomen or shoulder.
-
Serious allergic reactions: Anaphylaxis, a life-threatening allergic reaction, can occur. Symptoms include hives, swelling, and difficulty breathing.
-
Low platelet count (thrombocytopenia): This can increase the risk of bruising and bleeding. Platelet levels are monitored closely during treatment.
-
Leukocytosis: Plerixafor can significantly increase white blood cell counts.
Important warnings:
-
Plerixafor is not approved for use in patients with leukemia, as it may mobilize leukemia cells and contaminate the stem cell product.
-
The drug may be harmful to a fetus, and women of childbearing potential are advised to use effective contraception during treatment and for one week after the last dose.
Looking for "Plerixafor Injection" ?
Explore More Products