Chandkheda, Ahmedabad, Gujarat
- GST NO. : 24CJWPK9058H1ZI
Pharmaceutical Injections
Leading Manufacturers, Exporters, Wholesaler, Retailer and Trader of Benzathine Penicillin Injection, Ceftriaxone Injection, Cytoblastin Injection, Milrinone Lactate Injection, Octreotide Injection, Plerixafor Injection, Somatorel Injection, Somatropin Injection and Testosterone Enanthate Injection from Ahmedabad.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Grade Standard | Medicine Grade |
| Form | Liquid |
| Packaging Size | 5 Vales |
| Packaging Type | Yellow |
| Usage | Clinical, Hospital, Personal |
| Is It FSSAI Certified | Bottles |
| Generic Name | Penidure LA 12 |
| Dosage | As prescribed by doctor |
| Storage | Store in cool & dry place at room temperature |
| Pack Insert/Leaflet | Yes |
| Country of Origin | India |
| Type | Allopathic |
| Ingredients | Medical Grade |
| Brand Name | Benzathine |
| Product Code | Benzathine |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 5 vials in a box |
Benzathine Penicillin G is a long-acting form of the antibiotic penicillin, used to treat and prevent a wide range of bacterial infections. It is administered via a deep intramuscular (IM) injection, which allows the medication to be released slowly into the bloodstream over an extended period. This long-lasting effect makes it a valuable treatment for infections that require sustained antibiotic levels, often with a single dose.
Mechanism of Action
Benzathine penicillin G works by inhibiting the synthesis of the bacterial cell wall. It is a type of beta-lactam antibiotic that interferes with the cross-linking of peptidoglycans, a key component of the cell wall. This weakens the cell wall, eventually leading to the death of the bacteria.
Key Uses
This injection is primarily used for:
-
Syphilis: It is the first-line treatment for syphilis, especially primary, secondary, and early latent stages.
-
Strep Throat: It is effective in treating upper respiratory tract infections caused by susceptible Streptococcus bacteria.
-
Rheumatic Fever Prophylaxis: It is crucial for preventing the recurrence of rheumatic fever and rheumatic heart disease in patients with a history of strep infections.
-
Other Infections: It is also used to treat infections like yaws, bejel, and pinta.
Administration
-
Route: Benzathine penicillin is administered as a deep intramuscular injection, typically into the upper outer quadrant of the buttock in adults or the mid-lateral thigh in children.
-
Important Warning: This medication should NEVER be given intravenously (into a vein). Inadvertent intravenous administration can lead to serious and potentially fatal side effects, including cardiorespiratory arrest and nerve damage.
-
Pain: The injection is often thick and can be very painful. Healthcare providers may warm the vial to room temperature before administration to minimize discomfort.
Side Effects
As with any antibiotic, Benzathine Penicillin G can cause a range of side effects, from common to severe.
-
Common Side Effects:
-
Pain, swelling, or redness at the injection site.
-
Nausea, vomiting, or diarrhea.
-
Mild skin rash or itching.
-
Headache.
-
-
Serious Side Effects:
-
Allergic Reactions: Serious and sometimes fatal allergic reactions (anaphylaxis) can occur, especially in individuals with a history of penicillin or cephalosporin allergies. Symptoms include hives, swelling of the face, throat, or tongue, and difficulty breathing.
-
Jarisch-Herxheimer Reaction: In patients being treated for syphilis, a reaction may occur within the first 24 hours of treatment. Symptoms include fever, chills, headache, muscle aches, and worsening of skin sores. This is a sign that the medication is working and killing the bacteria.
-
C. difficile-associated diarrhea (CDAD): This is a serious intestinal condition that can occur during or even months after antibiotic treatment.
-
Neurological Side Effects: In rare cases, especially with very high doses or in patients with kidney problems, neurological symptoms like seizures, confusion, or hallucinations may occur.
-
Contraindications and Precautions
-
Hypersensitivity: The injection is contraindicated in patients with a history of hypersensitivity to penicillin or other beta-lactam antibiotics.
-
Renal Impairment: Caution is advised in patients with kidney disease, as the drug's excretion may be delayed, leading to higher levels in the body.
-
Pregnancy and Breastfeeding: While generally considered safe for use during pregnancy, it is always important to consult with a healthcare professional before use.
-
Drug Interactions: Benzathine penicillin may interact with certain medications, so it is important to inform the doctor about all other drugs being taken.
| Business Type | Manufacturer, Exporter, Supplier, Trader, Distributor |
| Medicine Type | Allopathic |
| Grade Standard | Pharm Grade |
| Usage | Pharmaceuticals |
| Form | Injection,Liquid |
| Strength | 200mg 500mg |
| Brand Name | Biocef-1g |
| Packaging Type | White,1 Vial |
| Certification | WHO GMP |
| Generic Name | Biocef-1g |
| Manufactured By | Leeford |
| Country of Origin | India |
| Product Code | Biocef-1g |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 1 Vial |
Ceftriaxone is a powerful antibiotic belonging to the cephalosporin class of drugs. It is administered as an injection, either into a muscle (intramuscularly) or a vein (intravenously), and is used to treat a wide variety of severe bacterial infections.
Key Information:
-
Active Ingredient: Ceftriaxone (in various strengths, such as 250 mg, 500 mg, 1 gram, or 2 grams).
-
Drug Class: Third-generation cephalosporin antibiotic. It works by inhibiting the synthesis of bacterial cell walls, which ultimately leads to the death of the bacteria.
-
Uses: Ceftriaxone injection is used to treat a broad spectrum of bacterial infections, including:
-
Serious Infections: Such as meningitis (infection of the membranes surrounding the brain and spinal cord), pneumonia, and sepsis (blood infections).
-
Urinary Tract Infections (UTIs).
-
Skin and soft tissue infections.
-
Bone and joint infections.
-
Sexually Transmitted Infections (STIs): Such as gonorrhea and syphilis.
-
Lyme disease.
-
Surgical Prophylaxis: It is often administered before certain surgeries to prevent post-operative infections.
-
-
Important Note: As with all antibiotics, Ceftriaxone is only effective against bacterial infections and will not treat viral infections like the common cold or flu. Its use is reserved for situations where an oral antibiotic may not be sufficient or for more serious infections.
Dosage and Administration:
-
Ceftriaxone injection is typically administered in a hospital, clinic, or by a trained healthcare professional. It is not self-administered at home unless specifically instructed and trained by a healthcare provider.
-
The dose and duration of treatment depend on the type and severity of the infection, and the patient's age and weight.
-
It is crucial to complete the full course of treatment as prescribed by the doctor, even if symptoms improve quickly, to prevent the development of antibiotic-resistant bacteria.
Common Side Effects:
-
Pain, tenderness, or swelling at the injection site.
-
Diarrhea (which can be a common side effect of many antibiotics).
-
Rash or itching.
-
Nausea and vomiting.
-
Abnormal liver function test results.
Serious Side Effects and Warnings:
-
Severe Allergic Reactions: A severe allergic reaction (anaphylaxis) is possible, especially in individuals with a known allergy to penicillin or other cephalosporin antibiotics. Symptoms can include a rash, hives, swelling of the face, lips, or tongue, and difficulty breathing. Seek immediate medical attention if these symptoms occur.
-
Gallbladder and Kidney Problems: In some cases, Ceftriaxone can cause gallstones or "biliary sludge," particularly in children, or affect kidney function.
-
Serious Diarrhea: While mild diarrhea is common, severe, watery, or bloody diarrhea may be a sign of a more serious condition called Clostridium difficile-associated diarrhea (CDAD), which can occur during or even months after antibiotic treatment.
-
Calcium Interaction: Ceftriaxone should never be mixed or administered in the same intravenous line with calcium-containing solutions, as this can lead to the formation of fatal precipitates in the lungs and kidneys. This is particularly dangerous in newborns and premature infants.
-
Pregnancy and Breastfeeding: It is important to inform your doctor if you are pregnant, planning to become pregnant, or breastfeeding.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Country of Origin | India |
| Composition | Vinblastine Sulphate Injection |
| Packaging Type | Vial |
| Grade | Medical Grade |
| Form | Liquid |
| Application | used to treat various types of cancer |
| Strength | 10 mg |
| Treatment | used to treat various types of cancer |
| Packaging Size | 10ml |
| Manufacturer By | Cipla Ltd |
| Generic Name | Cytoblastin |
| Type | Allopathic |
| Material | Medical Grade |
| Product Code | Cytoblastin |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 10ml vial |
Cytoblastin is a brand name for an injection containing the active ingredient Vinblastine. It is a powerful chemotherapy medication used to treat various types of cancer.
Drug Class and Mechanism of Action
Vinblastine belongs to a class of drugs called vinca alkaloids. These drugs are derived from the periwinkle plant (Catharanthus roseus).
Its mechanism of action is based on disrupting the cell division process. Specifically, vinblastine works by:
-
Inhibiting Microtubule Formation: It binds to a protein called tubulin, which is essential for forming microtubules. Microtubules are a key component of the cell's cytoskeleton and form the mitotic spindle, a structure necessary for cell division.
-
Causing Mitotic Arrest: By preventing the formation of the mitotic spindle, vinblastine stops cancer cells from dividing at a specific phase of the cell cycle (metaphase), which ultimately leads to the death of the cancer cells.
Uses
Cytoblastin Injection is used in the treatment of various cancers, either alone or in combination with other chemotherapy drugs. Its main uses include:
-
Hodgkin's Lymphoma and Non-Hodgkin's Lymphoma: Cancers that originate in the lymphatic system.
-
Testicular Cancer: A type of cancer that affects the testicles.
-
Breast Cancer: It can be part of a regimen to treat certain types of breast cancer.
-
Other Cancers: It may also be used to treat other conditions like Kaposi's sarcoma, mycosis fungoides (a type of cutaneous T-cell lymphoma), and some types of kidney and lung cancer.
Dosage and Administration
Cytoblastin is administered as an intravenous (IV) injection by a healthcare professional in a hospital or clinic setting. The dosage and frequency of administration are highly individualized and depend on the patient's specific condition, body surface area, and overall health. It is often given on a weekly basis as part of a chemotherapy cycle.
Side Effects
As a potent chemotherapy drug, Cytoblastin has several side effects. The most common and significant ones include:
-
Bone Marrow Suppression: This is a major concern, as it can lead to a dangerously low white blood cell count (neutropenia), increasing the risk of infections. It can also cause low red blood cell counts (anemia) and low platelet counts (thrombocytopenia).
-
Gastrointestinal Issues: Nausea, vomiting, diarrhea, constipation, and loss of appetite are common.
-
Hair Loss (Alopecia): This is a frequent side effect, though hair often grows back after treatment.
-
Neurological Effects: While less neurotoxic than some other vinca alkaloids, it can still cause nerve damage, leading to symptoms like numbness, tingling, or pain in the hands and feet (peripheral neuropathy).
-
Injection Site Reactions: Pain, redness, or swelling can occur if the drug leaks from the vein, as it is a vesicant (an irritant that can cause tissue damage).
Important Considerations
-
Pregnancy and Breastfeeding: Cytoblastin is known to cause harm to a developing fetus and is contraindicated during pregnancy. Women of childbearing potential should use effective contraception during treatment. It is also unsafe to use while breastfeeding.
-
Medical Supervision: Due to its powerful nature and potential for serious side effects, Cytoblastin must only be administered under the close supervision of a qualified oncologist.
-
Drug Interactions: Vinblastine can interact with other medications, so it is vital to inform the doctor about all other drugs, supplements, and herbal products being used.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Composition | Milrinone Lactate Injection USP |
| Packaging Size | 10ml |
| Grade | Medicine Grade |
| Usage | Clinical, Hospital |
| Form | Injection |
| Packaging Type | White |
| Generic Name | Milripro |
| Brand Name | Milripro |
| Type | Allopathic |
| Ingredients | Medical Grade |
| Country of Origin | India |
| Product Code | Milrinone |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 10 ML |
Milrinone lactate is a prescription medication administered as an intravenous injection, primarily used in a hospital setting for the short-term treatment of acute decompensated heart failure. It belongs to a class of drugs known as phosphodiesterase (PDE) inhibitors.
Mechanism of Action
Milrinone is a selective inhibitor of phosphodiesterase-3 (PDE3), an enzyme found in the heart and vascular smooth muscle. By inhibiting this enzyme, milrinone increases the concentration of cyclic adenosine monophosphate (cAMP) inside the cells. This leads to a dual effect:
-
Positive Inotropy: Increased cAMP in the heart muscle cells leads to a greater influx of calcium, which strengthens the force of heart muscle contraction. This improves the heart's pumping efficiency and increases cardiac output.
-
Vasodilation: In vascular smooth muscle cells, the increased cAMP levels cause the muscles to relax, leading to the widening of blood vessels. This reduces the resistance against which the heart must pump (afterload) and lowers the pressure in the veins returning to the heart (preload), further enhancing cardiac function.
Unlike some other heart medications, milrinone's effects are independent of beta-adrenergic receptors, which can be desensitized in patients with heart failure. This makes it effective in patients who may not respond to other therapies.
Indications
Milrinone lactate is indicated for the short-term intravenous treatment of patients with acute decompensated heart failure, where the heart's pumping function has become severely compromised. It is used to improve symptoms and hemodynamics in these critical situations, and its use is typically confined to intensive care units or cardiac units.
Dosage and Administration
Milrinone is a highly potent medication that must be administered intravenously under close medical supervision. The dosage is carefully calculated based on the patient's body weight and is typically given as:
-
Loading Dose: An initial dose administered as a slow push over a period of about 10 minutes.
-
Maintenance Infusion: A continuous intravenous infusion at a controlled rate, which is adjusted based on the patient's response and hemodynamic status.
The duration of therapy is generally short-term, as there is limited data to support its safety and efficacy beyond 48 hours. The dosage must be adjusted in patients with renal impairment to prevent drug accumulation.
Side Effects
The use of milrinone is associated with a number of potential side effects, with some being very serious. The most common and significant include:
-
Cardiac Arrhythmias: The most common adverse effect is an increase in ventricular arrhythmias (irregular heart rhythms), including ventricular tachycardia and ventricular fibrillation. This risk is particularly high in patients who already have underlying arrhythmias.
-
Hypotension: The vasodilatory effects of milrinone can cause a significant drop in blood pressure.
-
Headaches: Headaches are a common side effect.
-
Other Effects: Less common side effects include angina or chest pain, tremor, and thrombocytopenia (low platelet count).
Contraindications and Precautions
-
Hypersensitivity: Milrinone is contraindicated in patients with a known hypersensitivity to the drug or its components.
-
Severe Valvular Disease: It should not be used in place of surgical treatment for severe obstructive aortic or pulmonic valvular disease.
-
Acute Myocardial Infarction: The use of milrinone in the acute phase of a myocardial infarction is generally not recommended until further clinical experience is gained, as it may increase myocardial oxygen consumption.
-
Electrolyte Abnormalities: Low potassium levels (hypokalemia) can increase the risk of arrhythmias and should be corrected before milrinone administration.
-
Renal Impairment: Caution is required in patients with kidney disease, as the drug's half-life is significantly increased, necessitating a dosage reduction.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Medicine Type | Allopathic |
| Manufactured By | Sun Pharmaceutical Industries Ltd |
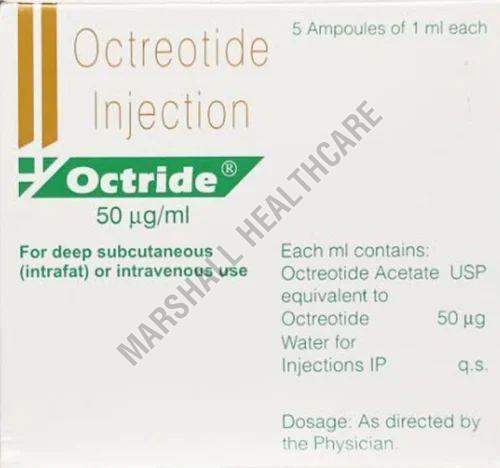
| Generic Name | Octreotide |
| Form | Liquid |
| Packaging Type | Paper Box |
| Composition | Octreotide50mcg |
| Country of Origin | India |
| Packaging Size | 1ml |
| Type | Allopathic |
| Product Code | Octreotide |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 1ml |
Octreotide Injection contains the active ingredient Octreotide, a synthetic version of a naturally occurring hormone called somatostatin. It is used to treat a variety of conditions, primarily those related to the overproduction of certain hormones.
Mechanism of Action
Octreotide mimics the actions of the natural hormone somatostatin, but it is more potent and has a longer duration of action. It works by binding to somatostatin receptors on the surface of cells, which then inhibits the release of various hormones from the body's endocrine glands, including:
-
Growth hormone (GH)
-
Insulin and glucagon
-
Thyroid-stimulating hormone (TSH)
-
Gastrointestinal and pancreatic hormones such as serotonin, gastrin, secretin, and vasoactive intestinal peptide (VIP).
By suppressing the secretion of these hormones, octreotide helps to manage the symptoms of various diseases caused by hormonal imbalances.
Uses
Octreotide injection is used for several key medical purposes:
-
Acromegaly: This is a disorder caused by the body producing too much growth hormone. Octreotide is used to reduce the amount of GH and its related substance, IGF-I, in patients who cannot be adequately treated with surgery or radiation.
-
Carcinoid Tumors: It is used to manage the severe diarrhea and flushing episodes that are common in patients with metastatic carcinoid tumors. These tumors can release excessive amounts of serotonin and other hormones.
-
VIPomas: It is used to control the profuse watery diarrhea caused by tumors that secrete vasoactive intestinal peptide (VIP).
-
Prevention of Pancreatic Surgery Complications: In some cases, it is used to prevent complications after pancreatic surgery.
-
Bleeding Esophageal Varices: It is used in the emergency management of bleeding esophageal varices (enlarged veins in the esophagus) in patients with cirrhosis. It works by reducing blood flow to the portal vein.
Types and Administration
Octreotide is available in two main forms for injection:
-
Short-Acting: This form is typically given as a subcutaneous (under the skin) injection, usually two to four times a day.
-
Long-Acting: This is a long-acting release (LAR) depot formulation that is given as an intramuscular injection into the buttocks, usually once every four weeks. This form is often used for long-term management after a patient has been stabilized on the short-acting form.
Side Effects
Common side effects of octreotide injection are often related to its effect on the gastrointestinal and endocrine systems. They can include:
-
Gastrointestinal issues: Diarrhea, constipation, nausea, abdominal pain, and flatulence.
-
Gallstones: The medication can alter gallbladder motility and bile secretion, increasing the risk of gallstone formation.
-
Injection site reactions: Pain, redness, or swelling at the injection site.
-
Blood sugar changes: Octreotide can affect blood sugar levels, causing either high blood sugar (hyperglycemia) or low blood sugar (hypoglycemia).
-
Cardiac effects: It can cause a slow heart rate (bradycardia) or other heart rhythm abnormalities.
Important Considerations
-
Medical Supervision: Octreotide is a powerful medication that must be administered and managed under the close supervision of a healthcare professional.
-
Monitoring: Patients on octreotide therapy will require regular monitoring of their hormone levels, blood sugar, gallbladder health, and cardiac function.
-
Drug Interactions: It is important to inform the doctor about all other medications, as octreotide can interact with several drugs, including insulin, oral diabetes medications, and certain heart medications.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Form | Celon |
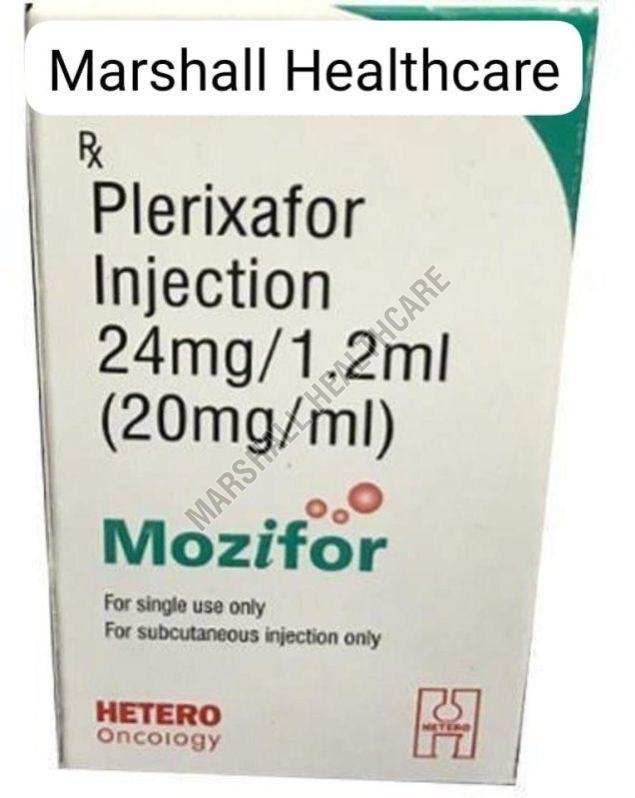
| Brand Name | Plerixafor Injection |
| Dosage Form | Injection |
| Grade | Medical Grade |
| Purity | 99% |
| Packaging Type | box |
| Composition | Plerixafor (24mg) |
| Packaging Size | 1 Injection in 1 Vial |
| Material | Medical Grade |
| Strength | 20MG/ML |
| Generic Name | Mozifor |
| Manufactured By | HETERO Oncology |
| Country of Origin | India |
| Product Code | Plerixafor |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 1 Injection in 1 Vial |
Plerixafor, sold under the brand name Mozobil, is a medication used in conjunction with other drugs to help mobilize hematopoietic stem cells (HSCs) from the bone marrow into the peripheral bloodstream. These stem cells are then collected from the blood and used for an autologous stem cell transplant.
Uses
Plerixafor is used in patients with certain types of cancer, specifically non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM). It is given as part of a regimen to prepare the patient for an autologous stem cell transplant, where the patient's own stem cells are collected and then reinfused after high-dose chemotherapy or radiation.
Plerixafor is used in combination with a granulocyte colony-stimulating factor (G-CSF) medication, such as filgrastim (Neupogen), which stimulates the bone marrow to produce more stem cells. Plerixafor's role is to help these newly produced stem cells move from the bone marrow into the bloodstream so they can be collected through a procedure called apheresis. This is particularly useful in patients who have a history of poor stem cell mobilization with G-CSF alone.
Mechanism of Action
Plerixafor is classified as a hematopoietic stem cell mobilizer and a CXCR4 antagonist. It works by blocking the binding of a protein called stromal cell-derived factor-1 alpha (SDF-1α) to its receptor, CXCR4, which is found on the surface of hematopoietic stem cells. The natural interaction between SDF-1α and CXCR4 is what anchors the stem cells within the bone marrow. By disrupting this interaction, plerixafor causes the stem cells to be released into the bloodstream, where they can be collected.
Administration
Plerixafor is administered as a subcutaneous (under the skin) injection by a healthcare professional in a medical facility. The injection is typically given once a day, approximately 11 hours before the apheresis procedure, for up to four days. The dosage is based on the patient's body weight and is part of a carefully managed treatment schedule.
Side Effects
As with any powerful medication, plerixafor can cause side effects. Some common side effects include:
-
Gastrointestinal issues: Nausea, vomiting, diarrhea, gas, and stomach pain.
-
Fatigue and headache.
-
Dizziness and joint pain.
-
Reactions at the injection site: Pain, redness, or swelling.
-
Vasovagal reactions: Some patients may experience fainting or lightheadedness, particularly shortly after the injection.
More serious, but less common, side effects can occur, including:
-
Splenic enlargement and rupture: Plerixafor, in combination with G-CSF, can cause the spleen to enlarge, and in rare cases, rupture. Patients are advised to seek immediate medical attention if they experience pain in the upper left abdomen or shoulder.
-
Serious allergic reactions: Anaphylaxis, a life-threatening allergic reaction, can occur. Symptoms include hives, swelling, and difficulty breathing.
-
Low platelet count (thrombocytopenia): This can increase the risk of bruising and bleeding. Platelet levels are monitored closely during treatment.
-
Leukocytosis: Plerixafor can significantly increase white blood cell counts.
Important warnings:
-
Plerixafor is not approved for use in patients with leukemia, as it may mobilize leukemia cells and contaminate the stem cell product.
-
The drug may be harmful to a fetus, and women of childbearing potential are advised to use effective contraception during treatment and for one week after the last dose.
| Business Type | Exporter, Supplier, Trader, Distributor |
| Composition | Recombinant Human Growth Hormone (Somatropin) Injection |
| Usage/Application | Primarily related to growth hormone deficiency and other growth-related issues |
| Storage | Cool & Dry Place |
| Function | Growth |
| Appearance | Colourless |
| Type | Allopathic |
| Purity | 100% |
| Dosage Form | Injection |
| Form | Liquid |
| Manufactured By | Reliance Life Science |
| Country of Origin | India |
| Material | Medical Grade |
| Brand Name | Somatorel |
| Product Code | Somatorel |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 1.5 ML |
Somatorel Injection is a medicine containing the active ingredient Somatropin, which is a form of recombinant human growth hormone (r-hGH). It is a hormone medicine that works by mimicking the body's natural growth hormone.
Uses:
Somatorel Injection is prescribed for various conditions, primarily related to growth hormone deficiency and other growth-related issues. It is used in:
-
Children with growth failure due to inadequate secretion of their own growth hormone (Growth Hormone Deficiency).
-
Children with short stature caused by specific genetic conditions such as Turner Syndrome and Prader-Willi Syndrome.
-
Children born small for gestational age who do not show catch-up growth by a certain age.
-
Adults to prevent severe weight loss related to AIDS and to treat short bowel syndrome.
-
Adults with growth hormone deficiency.
The injection helps in the metabolism of lipids, carbohydrates, and proteins, and is necessary for bone and muscular growth. It also promotes the proper development of fat and muscle tissues.
Administration:
Somatorel is administered as a subcutaneous injection (under the skin). A healthcare professional will typically administer the first dose and provide training on how to self-administer the injection at home. It's crucial to follow a doctor's instructions on dosage and administration and to rotate the injection site to prevent skin issues.
Potential Side Effects:
Common side effects of Somatorel Injection can include:
-
Pain, redness, swelling, or itching at the injection site.
-
Headache.
-
Joint or muscle pain.
-
Swelling of the hands, feet, or ankles (fluid retention).
More serious, but less common, side effects can occur. You should contact your doctor immediately if you experience:
-
Vision changes, severe headaches, nausea, or vomiting, which could be signs of increased pressure around the brain.
-
Limping due to hip or knee pain.
-
Signs of high blood sugar (e.g., increased thirst, frequent urination).
Important Safety Information:
-
Somatorel is a prescription medication and should only be used under the supervision of a healthcare professional.
-
Inform your doctor about any pre-existing medical conditions, such as diabetes, thyroid disorders, or cancer, as these can affect the treatment.
-
Do not use Somatorel if you are critically ill, have certain types of cancer, or have diabetic retinopathy.
-
It is generally not recommended for use during pregnancy or breastfeeding without consulting a doctor.
| Business Type | Manufacturer, Exporter, Supplier, Trader, Distributor |
| Usage/Application | Clinical, Hospital |
| Storage | Cool & Dry Place |
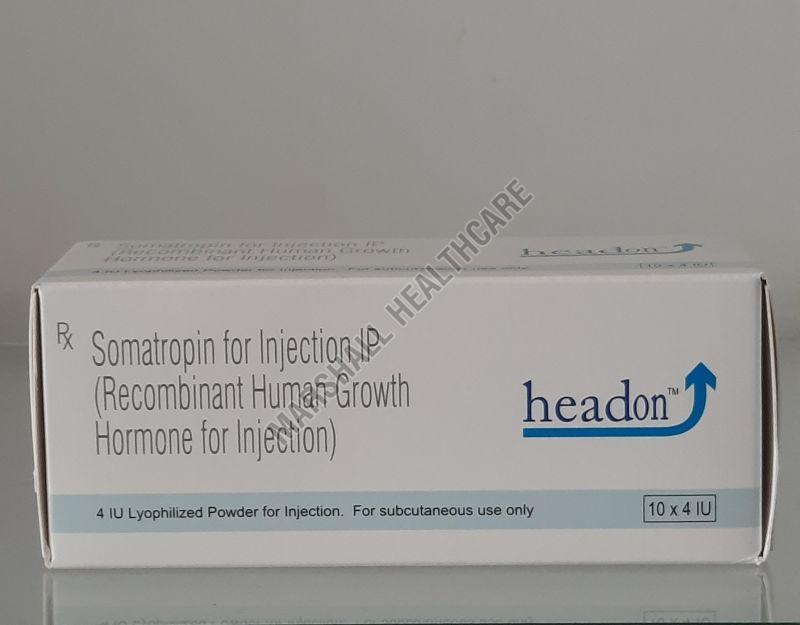
| Composition | Somatropin For Injection IP |
| Function | Growth |
| Appearance | Colourless |
| Color | Allopathic |
| Dosage Form | Injection |
| Form | Liquid |
| Generic Name | Headon |
| Brand Name | Headon |
| Type | Allopathic |
| Material | Medical Grade |
| Country of Origin | India |
| Product Code | Headon |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 1*1 vial |
Somatropin Injection is a man-made version of human growth hormone (HGH) that is used to treat a number of conditions in both children and adults. It is a potent hormone that plays a key role in growth, metabolism, and body composition.
Key Information:
-
Active Ingredient: Somatropin.
-
Drug Class: Growth hormone analog. It mimics the effects of natural human growth hormone produced by the pituitary gland.
-
Brand Names: There are many brand names for somatropin injection, including Genotropin, Humatrope, Norditropin, Nutropin AQ, Omnitrope, Saizen, Serostim, and Zomacton.
-
Uses: Somatropin is prescribed for:
-
Growth hormone deficiency (GHD) in both children and adults.
-
Growth failure in children due to conditions like chronic kidney disease, Turner syndrome, Prader-Willi syndrome, and idiopathic short stature (unexplained shortness).
-
HIV-associated wasting (cachexia) to increase lean body mass and improve physical endurance.
-
Short bowel syndrome (SBS) in adults who require nutritional support.
-
Dosage and Administration:
-
Somatropin is administered as an injection, typically under the skin (subcutaneously). Some preparations may be given into a muscle (intramuscularly).
-
The injection is usually given once daily.
-
The dosage is highly individualized and depends on the patient's age, weight, and the specific condition being treated.
-
It is crucial to follow a healthcare professional's instructions on how to prepare and inject the medication, as it may come as a powder that needs to be reconstituted with a liquid. Rotating injection sites is important to prevent skin changes.
Common Side Effects:
-
Pain, redness, swelling, or itching at the injection site.
-
Headache.
-
Muscle or joint pain.
-
Swelling in the hands or feet (fluid retention).
-
Nausea.
Serious Side Effects and Warnings:
-
Serious Allergic Reactions: A severe allergic reaction is possible. Symptoms can include a rash, hives, swelling of the face, tongue, or throat, and difficulty breathing. Seek emergency medical help if these symptoms occur.
-
Increased Risk of Cancer: There is a potential risk of developing certain types of cancer, including brain or skin cancer.
-
Increased Intracranial Pressure: The medication can cause increased pressure inside the skull, leading to severe headaches, vision problems, and nausea.
-
Diabetes: Somatropin can affect blood sugar levels and may cause or worsen diabetes.
-
Pancreatitis: Inflammation of the pancreas has been reported in rare cases.
-
Cardiovascular Effects: The medication can affect the heart and may cause heart rhythm problems.
-
Slipped Capital Femoral Epiphysis: In children, particularly those with growth hormone deficiency or Turner syndrome, there is a risk of a hip bone disorder. Call a doctor immediately if a child develops a limp or pain in the hip or knee.
Important Considerations:
-
Somatropin should only be used under the supervision of a doctor.
-
It is a powerful hormone that can have serious side effects if used improperly. Its use for non-medical reasons, such as bodybuilding or anti-aging, is illegal and dangerous.
-
Patients on this medication should have regular follow-up appointments with their doctor to monitor their progress and check for potential side effects.
| Business Type | Exporter, Supplier, Trader, Distributor |
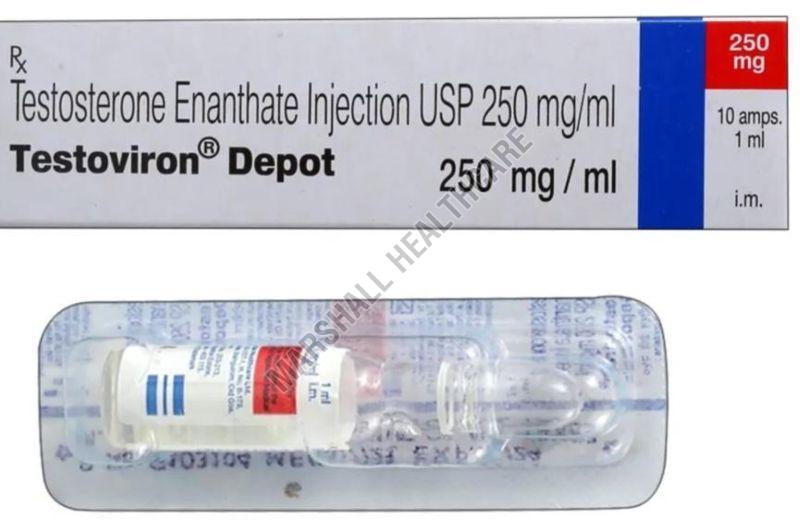
| Salt Composition | Testosterone Enanthate |
| Generic Name | Testoviron Depot |
| Storage | Cold & Dry Place |
| Color | Transparent |
| Strength | 250mg |
| Form | Injection |
| Medicine Type | Allopathic |
| API Form | Liquid |
| Country of Origin | India |
| Product Code | Testosterone Enanthate |
| Port | INAMD5 |
| Payment Terms | L/C, D/A, D/P, T/T, Western Union, Other |
| Delivery Time | 7 DAYS |
| Packaging Details | 10*1ml in a box |
Testosterone Enanthate is an injectable form of the male sex hormone, testosterone. It is a long-lasting medication that works by slowly releasing testosterone into the body. This makes it a popular choice for testosterone replacement therapy (TRT).
Uses
Testosterone Enanthate is primarily used to treat conditions in adult males associated with low or absent testosterone levels, a condition known as hypogonadism. Symptoms of hypogonadism can include:
-
Low sex drive
-
Erectile dysfunction
-
Infertility
-
Fatigue
-
Depressive moods
-
Loss of muscle and bone mass
Other approved uses for Testosterone Enanthate include:
-
Delayed puberty in boys.
-
Certain types of advanced, inoperable metastatic breast cancer in women.
-
Masculinizing hormone therapy for transgender men.
How It Works
Testosterone Enanthate is a testosterone ester, a form of testosterone that is modified with an enanthate molecule. When injected into the muscle, the oily solution acts as a depot, slowly releasing the testosterone into the bloodstream. Enzymes in the body then break down the ester bond, releasing free testosterone. This allows for a sustained and steady level of the hormone in the body for several weeks.
Administration
Testosterone Enanthate is typically administered by a healthcare professional as a deep injection into a muscle, usually in the buttocks. The frequency of injections can vary, but it is commonly given every 2 to 4 weeks. There are also subcutaneous auto-injector products available for self-administration, but their use is more limited and they are indicated for specific conditions.
Side Effects
As with any medication, Testosterone Enanthate can have side effects. It's important to discuss these with a doctor, as some can be serious. Common side effects include:
-
Injection site reactions: Pain, redness, or swelling at the injection site.
-
Hormonal and physical changes: Acne, increased body and facial hair, male pattern baldness, and an enlarged prostate (in men).
-
Cardiovascular risks: Increased blood pressure, fluid retention, and changes in cholesterol levels.
-
Blood-related issues: Increased red blood cell count (polycythemia), which can increase the risk of blood clots, heart attack, or stroke.
-
Reproductive issues: In men, it can suppress natural testosterone production, which can lead to a decreased sperm count and testicular atrophy.
-
Psychological effects: Mood swings, irritability, anxiety, and depression.
-
In women, use of testosterone can cause virilization, leading to a deepened voice, increased hair growth on the face and body, and changes in the menstrual cycle.
Important warnings:
-
Testosterone should not be used to improve athletic performance or for bodybuilding purposes due to the risk of serious health effects.
-
Testosterone Enanthate is a controlled substance due to its potential for misuse and dependence.